Making the Pain Go Away is the Only Thing On Your Mind
Avazzia Blue is a microcurrent electro-nerve stimulation device that stimulates the body’s natural responses to provide all-natural, drug-free, non-invasive OTC pain relief at home.


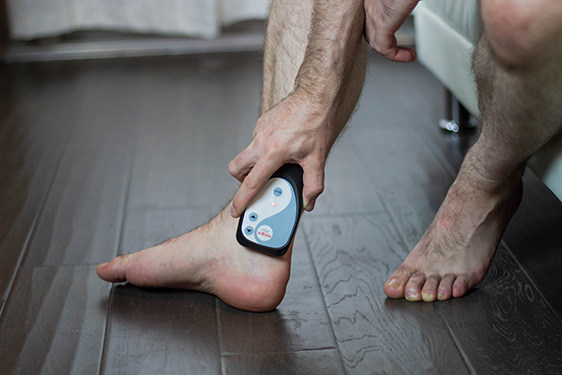
Avazzia Blue: Pain Relief Without Drugs
Avazzia Blue device is FDA-cleared for OTC pain relief. It is made with the same advanced neuromodulaton as prescription devices.
- Drug-free
- Non-invasive
- Simple to use – only two modes
- Cost-effective OTC pain relief
- Prescription-level pain relief without the prescription
Who Can Benefit?
- Relieves chronic and acute pain
- Chronic back pain
- Pain associated with sports (foot, tendon and calf pain)
- Plantar fasciitis and heel spurs
- Aches and pains caused by repetitive injuries (e.g., carpal tunnel syndrome)
- Pain associated with physical therapy (especially after knee or hip replacement)
- Back pain associated with travel or work
“After spending eight hours a day using a computer mouse, I began suffering from severe numbness in my right hand and arm. I was introduced to the Avazzia device and began seeing improvement almost immediately. Within six months I was totally symptom-free and able to avoid surgery.”




FAQs: Avazzia, Blue OTC Device, and More
What is it for?
The Avazzia Blue device is for OTC pain relief at home. It is a BEST™ microcurrent device.
Avazzia Bio-Electric Stimulation Technology (BEST) devices are FDA-cleared non-invasive, non-pharmacological neuromodulation devices for mitigating pain. These devices were developed to provide pain relief without surgery and without taking medicine. Results may vary depending on the source of pain, history of pain, other health issues, or adherence to treatment schedule. Even fatigue and hydration may impact outcomes. There are no known side effects. The technology does not interfere with the efficacy of other medicine or injections received for pain management. If you have more serious conditions causing your pain, ask your doctor to determine if Avazzia’s line of pain-management products might be suited for you. From time to time, information sessions are held throughout the year in various cities. You (or your healthcare provider) may attend an information session. Licensed practitioners are often on hand to talk about the benefits of Avazzia treatment. The Avazzia Blue™ device is available over-the-counter (no prescription required) for for pain due to exercise or normal daily activities. A prescription is required to purchase stronger Avazzia devices for chronic or intractable pain.
What are the benefits?
The Avazzia BEST devices have been cleared by the U.S. FDA, Health Canada, and have a CE safety certification as safe and effective OTC pain relief at home. Since BEST devices do not introduce drugs, medicine or toxins to your body, you will not suffer related potential side effects. Avazzia offers safe, cost-effective treatment. Patients have used Avazzia for the following reasons:
- They have found that traditional remedies for pain (drugs, surgery, physical therapy) are not helping or not helping enough.
- They do not want to take medication.
- They want to try something safe for nagging bothersome pain from doing normal, simple activities.
- They want the convenience of having something with them while they travel or at home for things like foot pain that hurts to walk or shoulder pain that hurts to raise their arm to comb their hair.
Does it work?
Many people report impressive results – significantly reduced pain and, frequently, pain completely eliminated. Healthcare practitioners, including medical doctors, dentists, chiropractors, physical therapists and athletic trainers, use Avazzia units for their own pain. A growing body of research shows that electro-stimulation effectively manages pain. Avazzia’s premier engineering ensures its units offer outstanding performance. Electro-stimulation is used by NASA, the U.S. military and hospitals, as well as rehab clinics, physical therapists and athletic trainers, to improve outcomes after injury and surgery. As with any medical device, results may vary from person to person based on individual circumstances.
What is electro-stimulation?
Electro-stimulation is the application of electrical impulses to the body. There are many types of electro-stimulation and several U.S. FDA classifications, such as:
- Heart stimulators (pacemakers)
- Implantable stimulators (frequently used for pain)
- Bone growth stimulators
- Transcranial stimulators
- Electrical muscle stimulators
- Transcutaneous (skin) electro-neurological stimulators (TENS): Avazzia falls into this category, and unlike many in this category, also falls into the subcategory of microcurrent. Avazzia BEST devices are unique in that they can detect changes in tissue reaction and electrical characteristics and can indicate when tissue stops changing.
How is Avazzia different than other, less-expensive TENS units on the market?
Avazzia devices are classified by the FDA as a TENS unit, as are possibly hundreds of other devices. Many of these TENS units haven’t changed their technology in decades. Avazzia technology was created in 2004 by a former Texas Instruments engineer to take the technology used in the 1970s into the 21st century. Key features of Avazzia’s BEST™ technology (Best Electro-Stimulation Technology) are its state-of-the-art engineering and that it is manufactured in the U.S.; its flexibility in addressing pain issues in patients; Avazzia’s outstanding customer service at the Dallas, Texas, headquarters; and its full training support both at in-person seminars and through online videos. Avazzia’s technology distinctions:
- Technology delivers more advanced output waveform and microcurrent.
- The interactive features and functions of Avazzia are patented and proprietary.
- The PRO-SPORT™ model gives a numeric reading of tissue reaction response data (Rx required for PRO-SPORT).
- Flexibility means all Avazzia devices can be used with any of the approved accessories or alone, with the onboard electrodes.
Because of the interactive response, the patient is less likely to develop accommodation or habituation from treatment. (Accommodation reduces the efficacy of almost every other TENS device on the market.) Devices are programmed to control the electrical amplitude (how much power is delivered); the frequency (the rate of electrical output) and other qualities such as burst pulse patterns; modulation; and damping.
What are your warranty and return policies?
Warranty
Avazzia BEST devices are sold with a limited one-year warranty against defect of material or workmanship.
Avazzia liability is limited to replacement or repair of product only, at manufacturer’s discretion. Warranty term is 12 months from the date of purchase. A receipt is necessary to validate warranty.
Lead wires, and personal items such as self-adhesive conductive electrode pads, conductive garments, and conductive wraps are non-refundable, non-returnable items.
IMPORTANT: For any repair that is outside of the warranty (by time or by scope, or due to damage), a cost estimation of repair will be issued. If you do not wish to accept the cost for repair, an inspection fee may apply.
The warranty becomes invalid when:
- More than one (1) year has passed since purchase.
- The unit was damaged or broken because of misuse or improper use.
- Rough handling.
- Water or chemical damage.
- Broken seal or case.
- Shorting of electrodes: The electrodes should not be directly shorted together (via metal, highly conductive liquids, etc.) while the device is on. This could damage the device.
- Electrostatic shock damage: Powerful electrostatic shocks into the electrodes may damage the device whether it is on or off.
- Violent vibration: Violent vibration could damage components and reduce the effectiveness of the device.
- Damaged accessory port due to misuse of accessory attachment misuse.
Returns
Refunds and Exchanges
Merchandise purchased from the Avazzia website for shipment to an address in the U.S. may be returned for credit or exchange within 30 days of purchase, subject to applicable restocking fees. Allow 14 days processing time once merchandise is shipped to Avazzia offices.
Customer is responsible for all shipping charges.
Restocking Fees
Returned electronic devices are subject to a 15 percent restocking fee.
Avazzia products are manufactured under stringent quality control in compliance with international quality standards and established safety guidelines.
In the event that a product gets broken or needs to be returned for service, repairs are performed by factory-trained staff in Dallas, Texas.
Avazzia purchases made outside of the United States should be returned to the authorized distributor or reseller for warranty support. Avazzia purchases made through an authorized distributor or reseller may also be returned to that distributor or reseller for warranty support.
For questions, call 214-575-2820 or click here to contact us.
How often may I use the device to treat my condition?
Follow your healthcare provider’s directions. It is not unusual to apply Avazzia microcurrent two to three times a day, for up to 20 minutes each time, for a week. After a week, many users find they significantly reduce their treatment time or frequency. Many discontinue daily use all together.
May I use this in addition to other drugs or after physical therapy sessions?
Yes. The only contraindications for using Avazzia technology are cardiac pacemakers and pregnancy. Make sure to read and follow warnings in the Owner’s Manual. And make sure your physical therapist knows you are using Avazzia.
May I take my Avazzia device through airport security?
Go to the Transportation Security Administration website for the latest information regarding batteries and battery-operated devices in carry-ons and checked luggage. No problems in traveling with Avazzia devices have been reported but the Federal Aviation Administration and TSA may change these regulations on very short notice.

Avazzia Blue
Avazzia Blue technology is the same advanced neuromodulaton that is built into prescription devices for pain relief including the same unique complex waveform with pulsed, high-voltage microcurrent streams.
- Prescription-level pain relief wtihout the prescription
- Drug free
- Non-invasive
- Microcurrent OTC pain relief medical devices for home use
- Simple to use – only two modes
- Cost-effective pain relief
- Advanced neurological signals to maximize pain relief
- Built-in, unique frequency-specific microcurrent pulse streams
The Avazzia Blue device uses 2 AA 1.5V batteries and has an accessory port for attaching lead wires and pads or electrode accessories.
The microcurrent modes in Avazzia Blue are designed for deeper therapy penetration:
- Blue Relax mode: Issues a gentle, constant pattern.
- Blue Stimulate mode: Issues a constantly changing pattern of pulses that ebb and flow like waves on a beach sometimes a little stronger, and sometimes a little more subtle.
Avazzia Blue technology provides temporary relief of pain associated with sore and aching muscles in the shoulder, waist, back, back of the neck, upper extremities (arm), and lower extremities (leg) due to strain from exercise or normal household work activities.
Special Offer: Avazzia Blue Kit OTC: Now $349.00 (+free shipping)
Blue Kit OTC includes Avazzia Blue Device, lead wires, 2″x2″ self-adhesive conductive gel pads (package of 4) and zipper pouch.
Avazzia Blue is an advanced microcurrent device for pain management for home use.
Prescription is not required. Avazzia Blue device not intended for diagnosis or treatment of disease. Not covered by insurance. Avazzia Blue device is US FDA cleared use for the temporary relief of pain associated with sore and aching muscles in the shoulder, waist, back, back of the neck, upper extremities (arm), and lower extremities (leg) due to strain from exercise or normal household work activities.
Warning: Not intended for individuals with pacemaker or other implanted electronic device or who may be pregnant.
MKT-130401-80A
